Zirconia powders process
High purity Zirconia (99.95% ZrO2) is required for producing partially stabilised zirconia (PSZ) ceramics which have impressive properties, allowing them to compete with metals and alloys for industrial applications. A process for the production of high grade zirconia powders was developed by a team from three CSIRO Divisions in collaboration with ICI Australia Ltd.
The team, led by Dr Hari Sinha (CSIRO Division of Mineral Chemistry) included Dr David Conochie, Dr Martin Houchin, David Jenkins, Ron McIntyre, Mary Woodward, Eric Caeser and John Moresby (all from the Division of Mineral Chemistry), Dr Gerry Haddad (CSIRO Division of Applied Physics) and Wolfgang Gross (CSIRO Division of Material Science).
The Zirconia powders process was licensed to ICI Australia Ltd and a pilot plant built, by ICI, at the CSIRO’s Mineral Chemistry site in Port Melbourne. The facility was to produce zirconia samples for market evaluation and provide design data for a full-scale plant. The process was highly successful and ICI Australia constructed the world’s largest zircon processing plant at Rockingham, WA. The production capacity of this plant was 700 tonnes per annum.
Historical Background
In 1957, CSIRO’s Ivan Newnham highlighted the anomalous position of Australian sheep skins being treated with solutions made from Australian zircon in the Upper Rhine region of Europe and suggested that Australian leather firms should carefully examine the French process. Newnham argued, why shouldn’t we prepare our own zirconium chemicals and tan our own sheep skins
In August 1964, the Division of Mineral Chemistry advertised for a partner to collaborate in the commercial development of the zirconia production process. The advertisement brought queries and expressions of interest from a number of firms ‘ particularly ICIANZ and Associated Minerals Consolidated ‘ but no contract eventuated. It is interesting to note that the revival of the work on the production of high purity zirconia in the 1980s resulted in an agreement between CSIRO and ICI Australia to commercialise the process as discussed below.
The process developed by CSIRO in the 1980s was quite different to the one researched earlier at the Division by Ivan Newnham and colleagues. It was based on sulphuric acid dissolution of the dissociated zircon, thus obviating the problem of the disposal of the sodium silicate solution produced in the caustic leaching of dissociated zircon.
Need for Research
Partially stabilised zirconia (PSZ) ceramics possess an impressive combination of properties which enable them to compete with metals and alloys in many applications where high temperature performance and erosion and corrosion resistance are required. Partially stabilised zirconia (PSZ) is much less brittle than other ceramics and is used for metal forming tools, valves and liners, abrasive slurries, kitchen knives, and bearings that are subject to severe abrasion. PSZ was developed by Garvie, Hannink, Pascoe and Nicholson from the CSIRO Division of Tribophysics in Melbourne and was first manufactured on a laboratory scale by Harold Kanost, an American ceramics engineer working in the same laboratory.
In order to produce zirconia ceramics for industrial applications, it is vital to produce zirconia powders of high purity (99.95% ZrO2), controlled particle size, morphology, surface area and weak agglomerate strength. One of the problems that hindered the development of PSZ, however, was the unreliability of the imported zirconia powders. It was in this context that Dr Hari Sinha was invited by Dr Mike Murray (who led the group working on PSZ), to a meeting with Drs Garvie and Hannink from the Division of Tribophysics, to explore the possibility of producing high purity zirconia for engineering ceramics applications. This resulted in the CSIRO Division of Mineral Chemistry initiating new research on the production of zirconia powders.
Early concerns
During the early stages of the research work CSIRO learnt that the Australian company, Associated Minerals Consolidated Limited (AMC) was planning to sell its zirconia plant, Ionarc Ltd, at Bow in New Hampshire, U.S.A. Since the thermal dissociation of zircon was part of the process being developed at CSIRO and the Bow plant had a plasma reactor to dissociate zircon, the purchase of this plant to be incorporated in the process development was considered. With the involvement of the Minister for Science, Mr Barry Jones, the CSIRO Executive met with Hari Sinha on 12th May 1983 and decided to send him to assess the Ionarc Ltd plant and advise on its purchase by CSIRO and the Australian Industrial Development Corporation (AIDC). As the matter was of utmost urgency, Dr Sinha flew out of Melbourne two days later, accompanied by Dr David Wilson of the (then) Commercial Group of CSIRO. Dr Sinha was chosen to make the evaluation because of his experience in process development research. His recommendation to purchase the plant was accepted by the Executive of CSIRO. However, the offer to AMC made through AIDC lapsed and the plant was subsequently sold to Ferro Corporation of U.S.A.
Success in six months
The research was rapidly concluded. Dr Sinha had returned from the plant at Bow with samples of both plasma-dissociated and caustic-leached zircon that contained 99.5% ZrO2. However, these were not pure enough for engineering ceramics. He promptly set about developing a new hydrometallurgical process to treat dissociated zircon and his patent to produce zirconia of 99.95% purity was filed in November 1983.
The high purity zirconia powder production process was as follows. Plasma dissociated zircon is ground to a particle size between 45 and 75 µm and heated with concentrated sulphuric acid at 320 ºC for 4 hours. Approximately 90% of the zirconia is extracted by this treatment. The unattacked silica is removed from the leach liquor by filtration. Zirconium sulphate crystals are obtained from the filtered solution by adding concentrated sulphuric acid until the sulphur trioxide concentration in the liquor is between 45 and 55%. High purity zirconia powder is obtained by calcining the zirconium sulphate crystals at temperatures of 900-1 000 ºC.
Hari Sinha recalled: The successful development of the above process within six months of starting the research was a matter of great satisfaction and elation. The next step was to find a suitable industrial partner to commercialise the process.
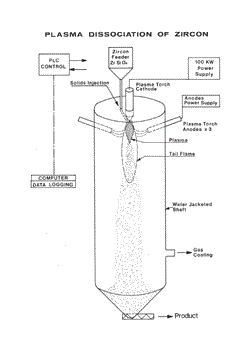
Commercialisation of the Zirconia Process
In December 1983, CSIRO advertised for an industrial partner to commercialise the zirconia process and an agreement was reached between CSIRO and ICI Australia Ltd. During the eighteen months which followed the signing of the agreement on 5th June 1984, each partner contributed about $1 million in research and development. A $0.5 million pilot plant was built by ICI Australia at the Division of Mineral Chemistry’s Port Melbourne site to produce zirconia samples for market evaluation and to provide design data for a commercial plant.
As the Bow plant for producing zirconia by the plasma dissociation of zircon had been sold to a foreign company, it was decided that the Division of Mineral Chemistry in collaboration with the Division of Applied Physics should design a plasma furnace to supply the dissociated zircon for the pilot plant. Dr Gerry Haddad from the Division of Applied Physics designed the plasma reactor and while it was built at Applied Physics, its installation at the Mineral Chemistry laboratories was done by Dr David Conochie and Mr Ron McIntyre. Before the CSIRO furnace had become fully operational, however, ICI Australia purchased the Ionarc plant at Bow; consequently all dissociated zircon for the pilot plant was supplied from this source.
Z-TECH Pty Ltd
In December 1985, ICI Australia Ltd and CSIRO, through its commercial agent, SIROTECH Ltd., set up a joint company, Z-TECH Pty Ltd, to penetrate overseas markets for high technology ceramic materials. Z-TECH was incorporated as a proprietary company, with ICI Investments Pty. Ltd and SIROTECH Ltd., holding shares in Z-TECH in the ratio of 85% and 15% respectively.
The agreement gave ICI Australia exclusive license to manufacture zirconia products in Australia and overseas. For the 3-year period following the formation of Z-TECH, CSIRO and ICI Australia agreed to commit $2.25 million each to further collaborative research and development. Also, under the agreement, CSIRO would withdraw from Z-TECH after the R&D phase had been completed. At this stage CSIRO would convert its interest into royalties paid on sales for 15 years.
Commercial Plant
On the basis of successful R&D and market research, ICI Australia built a 700 tonnes per year zirconia plant at Rockingham, WA at a cost of $13 million. David Jenkins, who was a member of the research team, spent some months at Rockingham helping to bring the plant on stream.
Honours and awards
For its Zirconia Powders Process, the joint CSIRO-ICI Australia team received the 1987 Ceramic Achievement Award from the Australian Ceramic Society. Dr Hari Sinha, who headed the CSIRO Mineral Chemistry team, was elected a Fellow of the Australian Academy of Technological Sciences and Engineering in 1986, and received the Sir Ian McLennan Achievement for Industry Award in 1987. Sinha was appointed a Member of the Order of Australia for services to science and technology and to Australian-Indian relations in 1990.
Source
- Sinha HN, 2009, Personal communication.
