Unravelling the structure of the wool fibre and other keratins
Almost immediately after the formation of the Biochemistry Unit of the Wool Textile Research Laboratories in the late 1940s, research began on unravelling the molecular structure of the wool fibre. Parallel investigations were conducted on three fronts: electron microscopy of the wool fibre by George Rogers; chemical studies on the number and nature of wool fibre proteins by Lennox and Gillespie, O’Donnell and Thompson, Crewther and Sparrow, Lindley and Elleman; and physical studies including X-ray and electron diffraction by Fraser, MacRae and Tulloch.
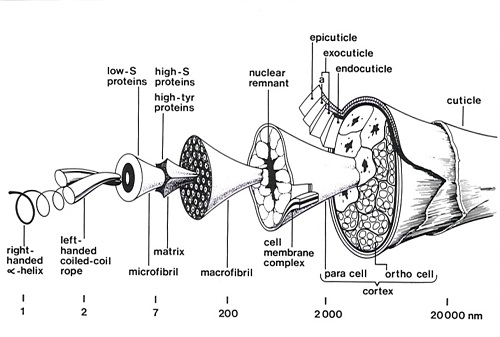
These studies revealed that the wool fibre was made up of a complex mixture of proteins of two main types: low sulphur proteins which are highly helical and are derived from the filamentous structures in the wool cell; and high-sulphur proteins which are non-helical and make up the matrix. The amino acid sequence data revealed that the low sulphur proteins are specialised intermediate filaments. Intermediate filaments, together with thin filaments (actin) and microtubules are important cytoskeletal elements that influence structural integrity, cell shape, and cell and organelle motility.
The combined EM, X-ray diffraction, electron diffraction and chemical data enabled a three dimensional model of the wool fibre to be assembled. The research included keratins other than wool as well as detailed X-ray diffraction studies of another fibrous protein collagen. The research is published in over 586 papers including 32 in the prestigious international journal Nature.
Background
Wool is a member of the keratin family of insoluble, proteinaceous, dead tissues which are rich in sulphur. They have been broadly classified as ‘hard’ or ‘soft’ according to their physical properties. The hard keratins – hair, wool, nails, beaks, claws, feathers, etc. – provide insulation, tools and weapons; the soft keratins, mainly epidermal structures, while playing a protective role may also have other important physiological functions. Keratins have been classified further as α-keratin, β-keratin or feather keratin according to their respective X-ray diffraction patterns.
Electron microscopy reveals wool fibre sub-structure
Earlier studies by Sikorski and Woods at Leeds and by Mercer, Farrant and Rees in CSIRO (Division of Chemical Physics) indicated that the cortical cells of the wool fibre could be disrupted into macrofibrils which are normally directed along the fibre axis. There was also evidence that the macrofibrils could be further disrupted into finer filaments, microfibrils.
On joining the laboratory in 1952, Bruce Fraser initiated studies on the infrared absorption of keratin and on the birefringence of stretched and normal fibres. He collaborated with George Rogers on the histology of the wool fibre obtaining information about its bilateral structure, its constituent membranes and its appearance after treatment with chemicals that rupture disulphide bonds. Studies were also carried out on the enhancement of fibre characteristics by means of shadow casting.
In the late 1950s, George Rogers was able to produce electron micrographs which demonstrated the ortho-para cortical segments, macrofibrils and microfibrils with superb clarity. The staining techniques which he used indicated that the matrix material between the macrofibrils and microfibrils is relatively rich in sulphur whereas the periphery of the microfibrils was relatively deficient in sulphur. It was also possible to discern some sub-structure in cross-sections of the microfibrils. The cortical cells in the wool fibre were shown to be of two different types. In merino fibres these cells have a bilateral distribution and constitute two adjacent strands known as the ortho and para segments which twist around one another along the length of the fibre so that the ortho segment is always found on the outside of each crimp wave.

Chemical studies
Wool proteins – a complex mixture of two types of helical proteins
Almost immediately after the formation of the Biochemistry Unit of the Wool Textile Research Laboratories, Gordon Lennox and Morton Gillespie commenced research on the extraction and fractionation of the proteins from the wool fibre. In 1953, Lennox and Gillespie published the first of an imposing series of papers on solubilised proteins from wool which could be separated into a low-sulphur fraction and a high sulphur fraction. Brian Harrap, using circular dichroism and optical rotatory dispersion, showed that the low sulphur protein fraction was highly helical in solution whereas the high-sulphur fraction was non-helical. This finding confirmed the existing hypothesis that the low sulphur proteins were derived from the filamentous structures in the wool cell and the high-sulphur proteins from the matrix.
A significant finding was Crewther and Harrap’s discovery that limited proteolytic digestion of the complete mixture of wool low-sulphur proteins gave rise to a particle that was highly helical in nature. This particle could be disaggregated and separated into two types where Type I was 60-80% helical and Type II was 18-36% helical. On mixing Type I and Type II peptides the helicity increased up to 120% greater than predicted whereas on mixing peptides of similar type no increase in helicity was observed.
The low sulphur proteins were shown by Ian O’Donnell and Ted Thompson, to contain 14 protein bands by starch gel electrophoresis with two of these bands, numbered 7 and 8 from the origin, predominant. In the 1970s, Lindsay Sparrow showed by polyacrylamide gel electrophoresis that both components 7 and 8 were themselves mixtures of several protein bands. The final conclusion was that there are: 8 low-sulphur proteins – four from component 7 (the Type II peptides) and four from component 8 (the type I peptides).
Low sulphur protein amino acid sequences
With the arrival of Ted Thompson in 1954, attempts were made to obtain amino acid sequence data from some of the fractions. Ted had just completed his PhD with Fred Sanger at the University of Cambridge. The papers by Sanger, Thompson and Tuppy on the amino acid sequence of insulin led to Sanger being awarded his first Nobel Prize for Chemistry in 1958. Although some terminal amino acid residues were identified the work was frustrated by the complexity of the proteins present, the fact that some of the N-terminal amino groups were blocked (acylated) and by the fact that the Edman method for the sequential N-terminal sequencing of proteins was still in its infancy and was not automated until the late 1960s.
The heterogeneity of the wool proteins hindered early attempts at obtaining sequence information and great effort was put into the development of fractionation methods. The purifications were carried out using very large columns (10 cm by 150 cm) of DEAE-cellulose onto which tens of grams of protein was loaded. These heroic efforts finally yielded pure protein components of members of each of the Type I and Type II families and their sequences were determined by a team led by Lindsay Sparrow and Gordon Crewther. Both types of helical proteins had a striking 7 residue pseudo-repeat with hydrophobic residues at the 1st and 4th positions of the heptad which conformed to the pattern for coiled-coil α-helical proteins predicted by Francis Crick in 1953. The Type I polypeptides were shown to be derived from component 8 and Type II polypeptides from component 7.
In the early 1980s, partial sequences for the proteins constituting intermediate-single filaments (IF) from various cell types appeared in the literature and it was immediately apparent that the microfibrils of wool were specialised IFs. The common factor in all IFs was the region predicted to form a coiled-coil α-helix. The disposition of these domains along all IF polypeptide chains was virtually identical suggesting a parallel arrangement of chains was required to maximise coiled-coil formation. Ionic interactions were also maximised by this arrangement.
The high sulphur (matrix) proteins
The high sulphur proteins were shown by Morton Gillespie also to be a complex mixtures of two classes of proteins: a fraction very rich in cystine; and a fraction with a high content of glycine (~40%), serine, tyrosine and phenylalanine which Gillespie designated the high-Gly-Tyr proteins. The final conclusion was that there were approximately 100 high-sulphur proteins (in four families of differing molecular weights); 15 high-glycine-tyrosine proteins (in two families) and an unknown number of ultra-high-sulphur proteins.
The first complete wool protein sequence from the Division was that of a high-sulphur protein by Tom Elleman. Three other sequences of high sulphur proteins from two different families followed. During the 1980s, as part of his PhD with George Rogers in Adelaide, Morry Frenkel determined the sequence of the cDNAs for three high-sulphur proteins as well as the sequence of a high sulphur protein gene and a pseudogene. In addition Mort Gillespie, Rob Marshall, Theo Dopheide and Adam Inglis sequenced three high-glycine-tyrosine proteins.
Bruce Fraser, Tom MacRae and David Parry identified a five-residue repeat and predicted β turns from the high sulphur protein sequences suggesting a highly crosslinked ‘firecraker’ structure for these proteins. On the other hand, Tom Elleman, Hugh Lindley and Bob Rowlands provided evidence that, though a five residue repeat was evident, a ten residue repeat was of greater significance.
Unfortunately, Tom Elleman’s high sulphur protein sequence was not the first wool protein sequence to be published; that honour went to Tom Haylett of the South African Wool Textile Research Institute who sequenced a member of a different family of high sulphur proteins. Haylett learned his sequencing craft from Hugh Lindley during a three year fellowship in the Division of Protein Chemistry.
Physical studies on keratins
In 1955, Tom MacRae joined Bruce Fraser in studies on the density of keratins, infra red absorption and dichroism, and hydrogen-deuterium exchange in keratins. At this stage, facilities were established at Parkville for X-ray diffraction studies to be applied to fibrous proteins. The collaboration between Bruce and Tom continued until both retired in 1987. Keeping in mind the protracted investigations needed by Perutz at this time for the determination of the structure of myoglobin using X-ray diffraction of crystals grown from highly purified protein samples, it is not surprising that the studies on keratin, a tissue containing heterogenous mixtures of several keratin protein families, should continue for more than three decades. As the early data accumulated, Bruce, Tom and various colleagues started theorising about the molecular orientation and configuration of α-keratins.
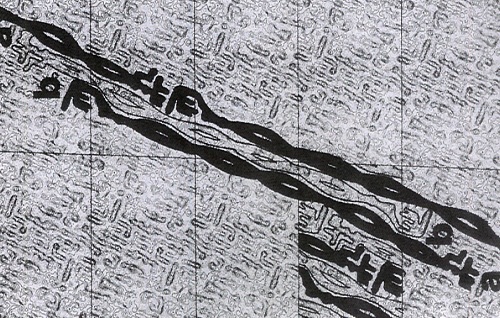
In the initial studies interpretation of the data was made difficult because of the fuzziness of many reflections. Consequently, difficulty was encountered in determining which areas were true meridional reflections and which included off-meridional arcs. Every effort was made to improve the clarity of the diffraction patterns and the experimental techniques for dealing with the data. To this end, Bert Jones, a skilled precision instrument maker, was assigned to the project and Bob Rowlands was established as software writer in the Division.
Over time they were able to refine their measurements of the electron density across the microfibril and with George Rogers, proposed a model for the microfibril consisting of a 7.5 nm filament with an outer annulus of α-helical protofibrils, some 2 nm in diameter, and a central core. Andrew Miller (a visiting Post doctoral Fellow from the UK), made a significant contribution to the quantitative calculations indicating that the model provided a plausible explanation for the observed X-ray diffraction data. The model was also in agreement with the structures observed in electron micrographs.
Improvements in camera design as well as the use of a focused X-ray beam provided improved primary data which were digitised and used to compute an averaged intensity transform of the specimen. This could then be displayed as optical densities on film using a digital analogue converter. In this way layer line spacings could be measured with greater precision. These data received considerable praise when presented internationally. In 1975, Bruce Fraser and Peter Tulloch, a brilliant young physicist who was pioneering electron diffraction techniques, were guest lecturers at the Summer School on Fibrous Proteins run by the European Molecular Biology Organisation and held at the University of Oxford. In a letter to Hugh Lindley, (the Acting Chief of the Division of Protein Chemistry at the time) Bruce wrote:
Tom [MacRae], Eikichi [Suzuki] and Bob [Rowlands] will be pleased to hear that the procedure developed at 343 [Royal Parade] for processing fibre diffraction patterns was a ‘show stopper’ and I was besieged by diffractionists both old and new for information on the method … Peter Tulloch’s talks were also greatly appreciated and as always there were queries about when he was going to publish details.
One evening a group of the students asked me to show them the night life of Oxford so I took them to the local disco, but the ‘bouncer’ took one look at Peter in his flared jeans and told him that we could only go in if we were decently dressed. Peter went back to college and returned with his all black ‘Al Capone’ outfit plus leather jacket but again the ‘bouncer’ stopped him saying “Sorry Sir, no bikies allowed”. Eventually the offending jacket was removed and we had an evening of 120+ decibels plus strobe lights etc. I haven’t been able to hear or see properly since.
A model is proposed
The data were found to fit a model for the microfibril in which units of α-helical coiled-coil rope are arranged on a helix of pitch 22 nm, with an axial rise per unit of 6.71 nm. After 7 or 8 such units a discontinuity occurs in the lattice, generating another helix of unit height 47 nm and unit twist 49.1°. Initially, calculations favoured 7 repeats before the mismatch in the lattice occurred but subsequent calculations of ionic interactions and disulphide bond formation using amino acid sequence data generated in the Division of Protein Chemistry by Lindsay Sparrow and colleagues, favoured 8 repeats.
Subsequently on the basis of the known sequence data for intermediate filaments (equivalent to the microfibrils of wool) Gordon Crewther, Lyndsay Dowling, David Parry and Peter Steinert (NIH, Bethesda USA) proposed a widely accepted model for the repeat unit in intermediate filaments (microfibrils). This model consisted of two Type I and two Type II chains forming two coiled-coil structures each with one chain of each type lying in parallel and in register. These coiled-coils then interacted in an antiparallel arrangement. The unit then consisted of a 4-chain central segment with a two-chain helical segment projecting from each end together with a non helical segment of chain. The units could be arranged on the lattice proposed by Fraser and MacRae for the microfibrils of α-keratin to give a model of the microfibril in the form of a telescope.
The structure of β-keratin
Bruce and Tom also proposed a preliminary model for β-keratin which is produced by stretching α-keratin in water. Progressive stretching of α-keratins, such as wool or hair fibres, in steam, leads to the appearance of new reflections in the equatorial pattern. These reflections are ascribed to interchain and intersheet spacings respectively in the pleated-sheet structures which are created during mechanical extension of the fibre. This β-keratin model was later refined (in association with David Parry and Eikichi Suzuki) by calculating the interaction energies between chains in alternative β-sheet arrangements and relating the expected spacings to those observed in the diffraction patterns. It was concluded that an anti-parallel chain arrangement was present. A later study provided an estimate of 25-30 polypeptide chains in each crystallite. This unit was equated to individual microfibrils.
Other keratins
Although most of the composition and sequence studies in the Division were carried out on wool, other keratins were also investigated, as models for wool. These included bird feathers, human hair, finger nail, animal hair (including that of an extinct mammoth found buried in a glacier in Alaska for 32 000 years), beaks, claws, animal hooves etc. In the case of human finger nail familial genetic factors could be identified and it was suggested that the techniques they used could answer questions relating to the taxonomy of birds. In association with Les Jones and Professor David Danks of the Royal Children’s Hospital research was conducted into abnormal human hair from children with genetic illnesses.
The widely accepted model for the keratin filaments developed by Bruce Fraser, Tom MacRae, Eikichi Suzuki and David Parry was based on data from the microfibril of porcupine quill. The stunning electron diffraction data obtained by Peter Tulloch was obtained using the otheca of the preying mantis (a model keratin). Peter Tulloch obtained electron density maps to 7 Å that are in excellent agreement with the model of Bruce Fraser and colleagues.
A model for collagen
The major focus on structural determination of collagen using X-ray fibre diffraction methods started in the early 1970s, and ultimately led to a detailed description for the conformation of the polypeptide chains in the collagen molecule, with the helical parameters fully defined. At this time, the nature of the bound water in collagen was still unclear. The availability of an accurate atomic model for the structure of collagen enabled further study, which in combination with data from more recent determinations of infrared dichroism, enabled models which incorporated hydroxyproline in the stabilisation of the water structure to be developed.
A major advance for the study of the unit cell and molecular connectivity of collagen came with the development of phosphotungstic acid treatment of tendons. The enhanced reflections which this method gave enabled a much better interpretation of the diffraction pattern and it was now possible to index many reflections in the various row-lines and layer-lines with confidence. As Bruce wrote to the Chief Gordon Crewther, during an overseas visit:
The electron diffraction pattern of Peter’s [Tulloch] that I showed got rave notices as usual. The work Tom [MacRae] and I did on the pitch of the molecular strands in collagen (P = 1 200 Å) aroused a lot of interest as Karl Piez has predicted the pitch from an analysis of the sequence and gets 1 200 Å. I’m sure that Tom will be pleased since so much work went in to getting that photograph.
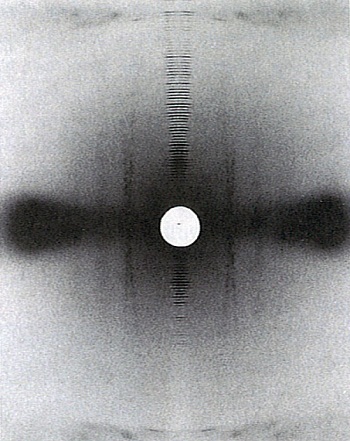
This improved data enabled further models for collagen packing to be examined, and led, in 1983, to a full description for collagen packing based on a quasi-hexagonal system. This paper was by Bruce, Tom MacRae, Eikichi Suzuki, in collaboration with Andrew Miller (UK). The earlier development of phosphotungstic acid staining to intensify the diffraction patterns proved an important advance for this work.
The data showed that the portions of the collagen molecules which contribute to the observed reflections have a common direction, with a length much less than the full molecule, and can be equated with the overlap zones in the packing scheme. Also, it showed that the paths of the individual molecules are not fully straight, with the molecule being crimped, and that the linking parts of the molecule, in the gap regions, are more mobile and vary in position along the fibril. The availability of this detailed structure for collagen packing enabled aspects of the relationship between amino acid sequence and packing to be examined, with the non-uniform distributions of certain amino acids being of particular interest in relation to the observed mobility of the gap region of the structure.
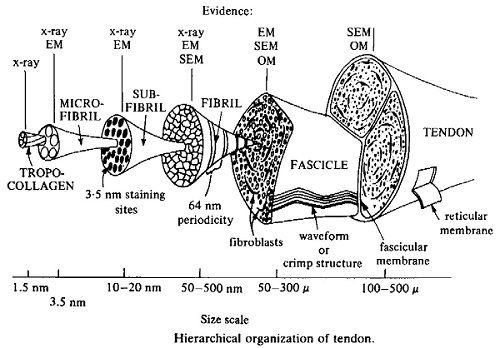
Impact on applied wool research
In Chapter 5 ‘Australian Innvation in Textile Technology’ of the book Technology in Australia 1788-1988 Don Taylor, former Chief CSIRO Division of Textile Industry stated:
In the early 1950s, the underlying thrust of the objective of CSIRO wool-textile research then -as now -was to tackle any technological weaknesses in the long chain of steps from the farm to the retail counter, and also to improve the attractiveness or marketability of the wool end-product to the ultimate consumer. There was also a strong belief that a better understanding of the complex chemical and physical structure of the wool fibre was fundamental to making progress in these two objectives. This meant that the nature of the research ranged from quite basic studies of fibre structure through to the development and proving of processes that could be easily adopted by industry.
By its very nature, this chapter is concerned with the latter type of activity, and hence the excellent fundamental studies carried out receive scant mention. This should not be taken to indicate that they have had little influence. On the contrary, although no one individual study can be highlighted as being the ‘breakthrough that changed wool science’, collectively these studies have built up a picture of the chemical and physical structure and properties of the fibre which is essential for the research scientists and technologists of today and the future.
Sources
- Rivett DE, Ward CW, Belkin LM, Ramshaw JAM, Wilshire JFK, 1996, The Lennox Legacy – A History of the CSIRO Laboratory at 343 Royal Parade Parkville, CSIRO Publishing, Melbourne, Australia, 370 pages.
- Taylor DS, 1988, ‘Chapter 5: Australian Innovation in Textile Technology’ , In: Technology in Australia 1788-1988, Australian Academy of Technological Sciences and Engineering, published by Australian Science and Technology Heritage Centre.
